CBD for Cyclists: Does It Really Work?
Over the last few years, CBD (Cannabidiol) has grown to become quite the acclaimed miracle antidote. And with benefits being touted for recovery and pain management (among other things), it’s even found its way into bikepacking and ultra-endurance sports. But does it really work? Here’s what we found out…
Note from the editor: A few years ago I had an acute back injury that I wrote about on this site. After surgery and during recovery (and a lot of physical therapy), I was determined to avoid the standard opioid medications that are often doled out for severe pain—and the side effects that come with them. I was immediately recommended CBD oil by a friend, which I was admittedly very skeptical about. But after having immediate results during periods of immense pain, I was interested in whether there was actually something to this “miracle drug.” During the first few weeks after the injury, I would use CBD several times per day, often when the pain became overwhelming or would keep me awake at night. To be honest, I was surprised when it worked. Virginia was even more of a naysayer than me, and with her career background in medical research, she began a deep dive into materials and studies that substantiate or disprove claims about CBD products. In the end, she put together this thorough article on the subject. It’s unlike most of the content we publish here, but I thought it was absolutely worth sharing. -Logan Watts
A Primer
Cannabis is an herbaceous plant that contains more than 500 components, of which 104 cannabinoids have been identified to date.1 Cannabinoids are chemical compounds or neurotransmitters that bind to cannabinoid receptors located throughout the body. Together, these endocannabinoids, their receptors, and enzymes make up the endogenous cannabinoid system, aka the endocannabinoid system. There is a ton of emerging science related to the topic, but what appears clear is that this system plays a crucial role in creating and maintaining homeostasis, or balance, within our bodies. It is involved in regulating a wide variety of processes, including memory, mood, appetite, pain, stress, metabolism, immune function, sleep, and reproductive function.
Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) are the most well-studied and widely familiar of these cannabinoids. THC is the primary psychoactive compound in cannabis. CBD is the second most prevalent of the active ingredients of cannabis and, by itself, does not cause a “high.” CBD has been touted by some supplement manufacturers as a cure-all that treats conditions including inflammation, chronic pain, opioid withdrawal, depression, autoimmune disorders including diabetes, neurodegenerative disorders including Alzheimer’s and Parkinson’s diseases, metabolic disorders that lead to obesity, and even cancer. Of particular interest to athletes is CBD’s purported benefits in reversing oxidative stress and treating inflammation, both of which support muscle recovery.

What Does the Science Actually Support?
The claims that individual manufacturers of CBD products make have not been substantiated by the US Federal Drug Administration (more on that to come), but there has been—and continues to be—a great deal of clinical research that focuses on CBD. At the time of writing, a simple search of “cannabidiol” on clinicaltrials.gov, which catalogs medical studies on human volunteers, shows that 73 clinical trials (interventional) have been completed to date, while 100 studies are currently active.2 The total number of CBD-specific human trials has been somewhat limited due to legal issues surrounding cannabis and the lack of profitability perceived by big pharma, but a number of these completed trials have proven that CBD alone is beneficial in treating at least two devastating seizure disorders.3 When combined with THC in a 1:1 ratio, CBD has also been proven to ameliorate some of the worst symptoms associated with multiple sclerosis (MS), like neuropathic pain, spasticity, and muscle spasms4. The combination has also been proven to treat severe neuropathic pain (pain caused by damage to or diseases affecting the nervous system) in cancer patients5. While clinical trials in the US are still in their relative infancy, more than a dozen countries already have CBD-based medications on the market.
Biomedical research studies based on animal models have been far more numerous than human clinical trials. Much of the research shows promising results for the potential use of cannabinoids in the treatment of a host of human medical conditions, including diabetes, psychiatric, neurodegenerative, and gastrointestinal disorders. In regards to pain, research conducted thus far, along with countless user reports, suggests that CBD alone may be able to help relieve both inflammatory and neuropathic pain in humans. As just one of many examples, a paper in the European Journal of Pain revealed that rats with arthritis who are treated with transdermal CBD experienced reductions in pain-related behaviors and inflammation6. These animal-based studies are helping to further our knowledge of cannabis’ potential medical benefits. More importantly, they are opening the door for more human-based research and clinical trials to be conducted down the line.
Legality
Cannabis is one of several genera of flowering plants in the family Cannabaceae. The number of species within the genus is up for debate. Depending on the rules of taxonomy that are followed, there is either one highly variable species, Cannabis sativa, or there are three distinct species, C. sativa, Cannabis indica, and Cannabis ruderalis. Regardless of one’s stand on the nomenclature debate, it is helpful to understand that both hemp and cannabis with psychoactive constituents (aka marijuana, ganja, weed, reefer, sinsemilla, etc.) come from the same species, C. sativa, and, until recently, the two were treated the same in terms of their legality in the United States. When the Controlled Substances Act was signed into law by Richard Nixon in 1970, all cannabis varieties “whether growing or not; the seeds thereof; the resin extracted from any part of such plant; and every compound, manufacture, salt, derivative, mixture, or preparation of such plant, its seeds or resin…” were classified as Schedule I controlled substances (along with heroin, Quaaludes, GHB, MDMA, and LSD among others). Schedule I controlled substances are defined as drugs with “no currently accepted medical use” and a high potential for abuse.7
The Agricultural Act of 2014, more commonly known as the 2014 Farm Bill, provided the first legal distinction between psychoactive cannabis and hemp. Industrial hemp was defined as “the plant Cannabis sativa L. and any part of such plant, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis.” The law legalized the growth and cultivation of industrial hemp for research purposes under an agricultural pilot program.8 Still, it wasn’t until the Agriculture Improvement Act of 2018 was signed into law that the production of hemp as an agricultural commodity became legal nationwide, and it was removed from the DEA’s list of controlled substances.
Along with hemp itself, CBD derived from hemp (not that derived from cannabis with greater than 0.3% THC) is federally legal in all 50 states and is no longer under the jurisdiction of the Drug Enforcement Agency (DEA). Additionally, in January 2019, the World Health Organization made its recommendations regarding CBD, stating that “preparations considered to be pure cannabidiol (CBD) should not be scheduled within the International Drug Control Conventions.”9 In terms of its use by competitive athletes, CBD has been removed from the World Anti-Doping Agency’s (WADA) list of banned substances since 2018.

Is CBD a Nutritional Supplement, a Drug, or What?
While CBD is federally legal, that does not mean it is free from oversight. In the United States, the Food and Drug Administration (FDA) “is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices; and by ensuring the safety of our nation’s food supply, cosmetics, and products that emit radiation.”10
So, under which of these categories does CBD fall? The answer to that question is an elusive one. In 2018, the FDA approved a CBD-based medication, Epidiolex, for the first time. With that drug approval, CBD moved into new gray territory. The FDA says that because CBD is also an approved drug (Epidiolex), the cannabinoid cannot be considered, as many argue it should be, a dietary supplement.
This is where things get particularly foggy. All medications, whether over-the-counter or prescription, must be proven safe and effective before the FDA approves them, while dietary supplements do not have to meet these standards. The FDA regulates dietary supplements as food and can only force a company to stop making them if they can prove that the product poses a significant risk to people’s health. Since supplements do not receive prior approval from the FDA, they can only shut down a supplement manufacturer after injury has been caused. The other way in which the FDA can regulate a supplement is by “taking action against any adulterated or misbranded dietary supplement product after it reaches the market.”11
While the FDA considers CBD a drug, they have, thus far, treated it more like a supplement in terms of regulation. The FDA has yet to force any testing standards on manufacturers of CBD products. They are aware that these CBD products have not undergone rigorous clinical trials, yet products remain available to consumers. What the FDA has done, however, is crack down on manufacturers for making unsubstantiated claims about their products. The FDA went a step further in its admonition to CBD product manufacturer Curaleaf, Inc., stating in its warning letter dated 22 July, 2019, that a number of their products are unapproved and misbranded “new drugs” and that “there are many unanswered questions about the science, safety, effectiveness, and quality of unapproved products containing CBD.”12 Curaleaf, Inc. was ordered to correct stated violations or face legal action without further notice.
So, as it currently stands, CBD dietary “supplements” are illegal under the FDA’s rules, but it is not federally illegal to be in possession of such products. The FDA is exploring ways to legally get CBD products to market, but in the meantime many manufacturers are giving their hemp-derived CBD products a new name, “broad-spectrum hemp extract,” to avoid FDA sanctions.

Potential Risks
With so much evidence suggesting that CBD can be effective in treating a host of human conditions, it is no surprise that its popularity is soaring. Unfortunately, as is the case with most good things, there are potential risks associated with its use.
Dangers specifically associated with vaping have been making big news of late. In light of the recent surge in morbidity and mortality related to vaping, The Centers for Disease Control and Prevention (CDC) is recommending that people should not use THC-containing e-cigarette or vaping products, particularly those from informal sources like “friends, or family, or in-person or online dealers.” They have also added that “the only way to assure that you are not at risk while the investigation continues is to consider refraining from use of all e-cigarette, or vaping products.”13
Aside from recent news highlights, the greatest risks of CBD actually come from lack of regulation. As is the case with the countless herbal and dietary supplements that line the shelves of your local pharmacy, there are no guarantees that the CBD product a consumer purchases actually contains the ingredients a manufacturer claims or has the potency they advertise. In fact, a 2017 study published in The Journal of the American Medical Association found that 69 percent of the CBD products tested did not contain the amount of cannabidiol indicated on the label.14 Another concern with all of these unregulated products is that of purity. Numerous CBD products have tested positive for heavy metals, like lead and mercury, as well as pesticides and microbiological contamination.
Additionally, most CBD products have not been tested to determine proper dosage, how they could interact with FDA-approved drugs, or whether they have dangerous side effects. One specific risk potentially associated with CBD use is that of liver toxicity. In fact, Epidiolex’s label includes a warning about that risk, and the manufacturer recommends that prescribing physicians monitor patients’ liver enzymes. That said, dose adjustments are only recommended for patients with moderate to severe liver impairment, not for those with mild liver impairment.15
It appears that the FDA will be moving toward tighter regulation regarding CBD-based products. Increased monitoring and regulation of all dietary supplements is needed to make them safer for the public, but to single out CBD-based products seems curious. Given the fact that any number of unregulated dietary supplements come with a much higher risk of toxicity, it is highly debatable as to whether such a move would have as much to do with public welfare as it does with bolstering pharmaceutical industry profits.
Products and Methods
Now that we’ve covered the legal and regulatory issues that surround CBD products, let’s take a closer look at how they are consumed and the benefits and/or disadvantages of each route of administration. For each of the methods listed below, there are numerous products that can be used. Please note, this product list is not exhaustive, as new products are coming to market at a dizzying pace.
ORAL (swallowed and digested by the gastrointestinal system)
Common ingestible CBD products include capsules, edible candies and chocolates, as well as powders for mixing into beverages and foods.

TRANSMUCOSAL (absorbed via mucous membranes)
Transmucosal routes of administration are further broken down by the location of the membranes being utilized. Oral transmucosal are either buccal (cheek) or sublingual (under the tongue). Products designed to work buccally include lozenges and chewing gums. Sublingual products include oil-based tinctures in the form of drops and sprays. For the nasal mucosa, there are aerosol sprays, and CBD suppositories are available for both rectal and vaginal administration.
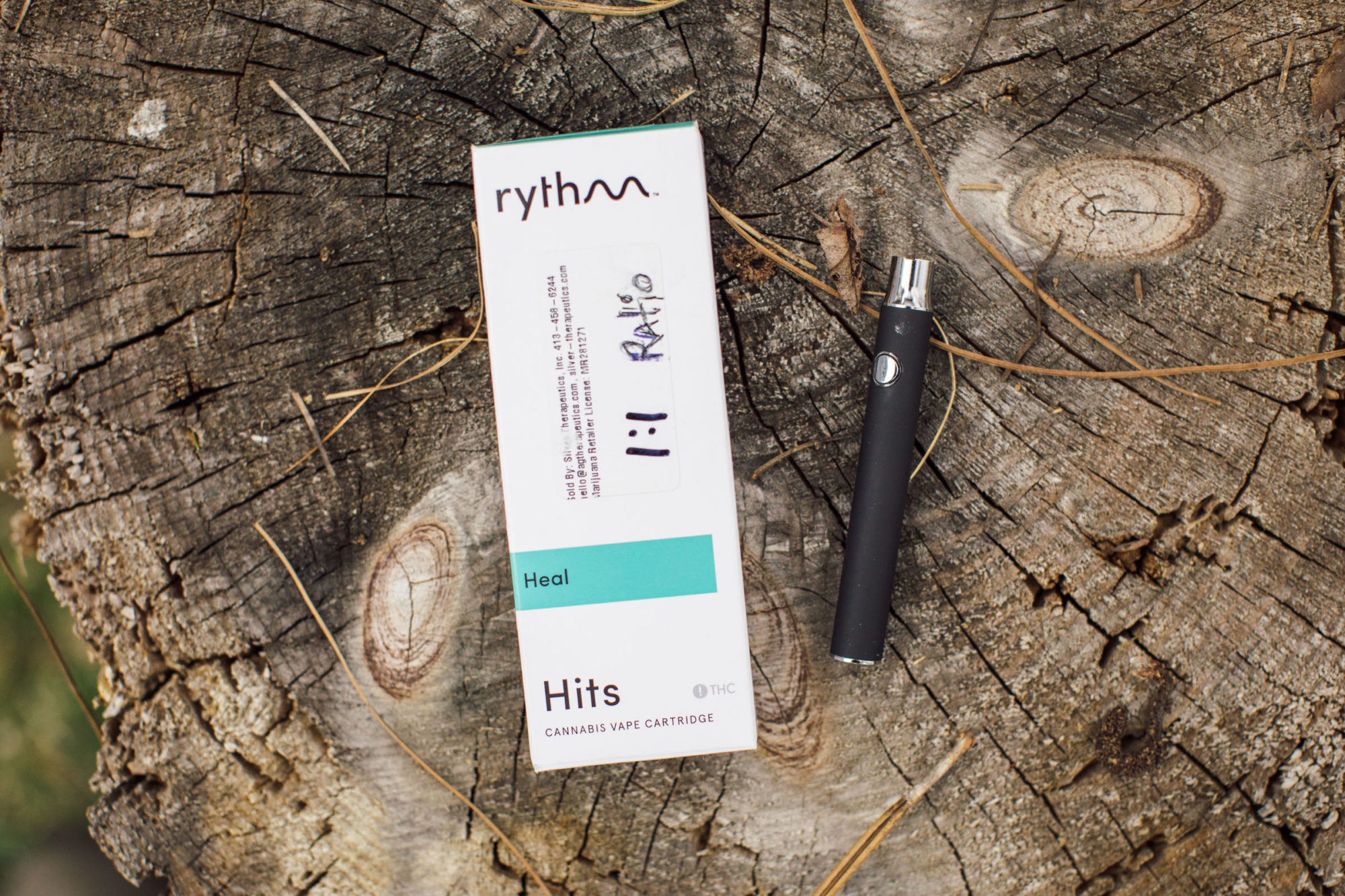
PULMONARY (inhaled and absorbed via the lungs)
Aerosolized distillate inhalers and oils for vaporizers or pre-filled vape pen cartridges as well as traditional “buds” are all products available for inhaling CBD.

CUTANEOUS (absorbed through the skin)
The cutaneous routes of CBD administration include both topical and transdermal applications. CBD products intended for topical use include ointments, creams, salves, and balms. Topicals do not absorb into the circulatory system, and, therefore, do not have a systemic (whole body) effect. Transdermal patches contain a mixture of CBD and a carrier that allows the CBD to penetrate through the skin and into the circulatory system.
Dosing
Unfortunately, there aren’t any hard and fast rules related to CBD dosing. There are a host of variables that come into play, including the condition(s) being treated, an individual’s body mass, their metabolism, age, and the mode of CBD transmission being utilized, as well as the composition of the CBD product being used. There are several online CBD dosage calculators available, such as https://cbddosagecalculator.com/start/, but it is difficult to find hard research that supports the guidance they provide. Most manufacturers of CBD products do provide dosing guidelines on their products’ packaging, but it is important to remember that those guidelines are specific to a particular packaged product, as different products come in varying strengths or potencies and even have different constituents.
A good rule of thumb is to start at a low dose and increase the dosage on a consistent basis until relief is obtained, remembering to never exceed recommended daily dosages. This is called titration. An example of medication titration can be found in the literature that accompanies a Sativex prescription.16 Please note, Sativex is a 1:1 THC/CBD medication (non-FDA-approved), so these guidelines are not intended for stand alone CBD dosing. It is also recommended that CBD be used on a consistent basis for best results, especially when chronic conditions are being “treated”. By taking CBD at a “scheduled” time or times of day, a more stable level of CBD is in the blood over a longer span of time.
Some science also suggests that finding the therapeutic range of a full-spectrum CBD product (one with a range of cannabinoids in addition to CBD as well as terpenes) may be easier when compared to a CBD-isolate. 17
CBD/Hemp Buying Tips
There are a lot of junk products on the market, so experts suggest that you follow some simple steps if you intend to purchase and use CBD goods. Here are just a few tips that you can follow that may steer you in the direction of safer and potentially more efficacious products.
- Only purchase products that have been tested by independent, third-party laboratories that are ISO/IEC 17025 accredited.
- Ask to see a product’s COA (certificate of analysis). If that information is not available, shop elsewhere or purchase a different product. The COA will include data on the CBD and/or THC concentrations of the tested product as well as the results of heavy metals/purity tests.
- Make sure the product you purchase includes vital information, including the volume of CBD per serving.
- Find out where the hemp was grown.
- If possible, purchase CBD products from distributors located in “marijuana” legal states, as these producers are often subject to more oversight than those in other states.
- Make sure products are in dark containers. You may also ask about their storage conditions, since temperature-controlled environments are advised.
Final Thoughts
There is a lot of quality research that supports the use of CBD and broad-spectrum hemp products as an adjunctive treatment for a range of health conditions, including inflammation and pain. However, there are also a lot of products on the market that make unsubstantiated, miracle-cure claims. It is important for consumers to be well informed and diligent in their own research. It is also important that the scientific community continue its research, so that we can have a better understanding of cannabis’ full potential in the preservation of human and animal health as well as the treatment of disease.
Editor’s Note: As mentioned in the intro, I found pain relief with CBD oil such as the “Plus” brand we researched, as shown in some of the photos above. I’ve also tried a couple others, as well as the products shown by Floyd’s of Leadville. I really liked the recovery mix; it tastes great and contains a fair amount of protein as well as CBD. I’m not 100% sure on the efficacy of ingesting CBD extract. Oil may be a little more effective in that regard. However, it did seem to have a relaxing effect, which was nice after a long ride. -Logan
Disclaimer: Information provided in this article and in any materials referenced in it are not guaranteed or warranted to be accurate or complete. Use or dissemination of any of the information in this article is at your own risk. Among other risks, the user of this site assumes the risk that any herb, dietary supplement, or medicine may be ineffective, may cause harmful effects, reactions or interactions, or may cause, contribute to, or exacerbate temporary or permanent bodily injury, sickness, or other harm, including death.
2 Find Studies. (n.d.). Retrieved January 7, 2020, from https://clinicaltrials.gov/ct2/search/index.
3 Silvestro, Serena, et al. “Use of Cannabidiol in the Treatment of Epilepsy: Efficacy and Security in Clinical Trials.” Molecules, vol. 24, no. 8, 2019, p. 1459., doi:10.3390/molecules24081459.
4 Koppel, B. S., et al. “Systematic Review: Efficacy and Safety of Medical Marijuana in Selected Neurologic Disorders: Report of the Guideline Development Subcommittee of the American Academy of Neurology.” Neurology, vol. 82, no. 17, 2014, pp. 1556–1563., doi:10.1212/wnl.0000000000000363.
5 Blake, A, Wan, BA, Malek, L. A selective review of medical cannabis in cancer pain management. Ann Palliat Med 2017; 6: S215-S222.
6 Hammell, D C, et al. “Transdermal Cannabidiol Reduces Inflammation and Pain-Related Behaviours in a Rat Model of Arthritis.” European Journal of Pain (London, England), U.S. National Library of Medicine, July 2016, www.ncbi.nlm.nih.gov/pubmed/26517407.
7 https://www.govinfo.gov/content/pkg/STATUTE-84/pdf/STATUTE-84-Pg1236.pdf#page=57.
8 2014 Hemp Farm Bill Section 7606. (n.d.). Retrieved January 7, 2020, from https://www.votehemp.com/laws-and-legislation/federal-legislation/sec-7606-of-the-farm-bill/.
9 Ghebreyesus, T. A. (n.d.). Retrieved January 7, 2020, from https://www.who.int/medicines/access/controlled-substances/UNSG_letter_ECDD41_recommendations_cannabis_24Jan19.pdf?ua=1.
10 Commissioner, Office of the. “What We Do.” U.S. Food and Drug Administration. FDA. Accessed July 5, 2019. https://www.fda.gov/about-fda/what-we-do.
11 Center for Food Safety and Applied Nutrition. “Dietary Supplements.” U.S. Food and Drug Administration. FDA. Accessed July 7, 2019. https://www.fda.gov/food/dietary-supplements.
12 Ashley, Donald D. “Curaleaf, Inc – 579289 – 07/22/2019.” U.S. Food and Drug Administration. FDA, July 23, 2109. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/curaleaf-inc-579289-07222019.
13 “Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products.” Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, November 26, 2019. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html.
14 Ashley, Donald D. “Curaleaf, Inc – 579289 – 07/22/2019.” U.S. Food and Drug Administration. FDA, July 23, 2109. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/curaleaf-inc-579289-07222019.
15 Greenwich Biosciences, Inc. (2018). Epidiolex: Highlights of Prescribing Information. Epidiolex: Highlights of Prescribing Information. Carlsbad, Ca.
16 “Sativex Oromucosal Spray.” Sativex Oromucosal Spray – Summary of Product Characteristics (SmPC) – (emc). GW Parma Ltd. Accessed September 5, 2019. https://www.medicines.org.uk/emc/product/602/smpc.
17 Gallily, R., Yekhtin, Z., & Hanuš, L. O. (2015). Overcoming the Bell‐Shaped Dose‐Response of Cannabidiol by Using Cannabis Extract Enriched in Cannabidiol. Pharmacology and Pharmacy, 6, 75–85. Retrieved from https://file.scirp.org/pdf/PP_2015021016351567.pdf
Please keep the conversation civil, constructive, and inclusive, or your comment will be removed.